The FDA has just approved zongertinib (brand name Hernexeos) – a new targeted pill – for adults with advanced non-small cell lung cancer (NSCLC) that carries a specific HER2 gene mutation. This approval applies to patients whose cancer is “non-squamous” (usually adenocarcinoma type) and who have already received at least one prior therapy. In practical terms, that means zongertinib is for lung cancer patients with a rare HER2 mutation (in the kinase domain of the HER2 gene) after standard chemotherapy or other treatments. Roughly 2–4% of NSCLC tumors have these HER2 mutations. Until now, treatment options for these patients were very limited, so this FDA action brings new optimism.
Zongertinib works by selectively targeting the HER2 protein that fuels tumor growth, while sparing a related protein (EGFR) that often causes side effects like rash and diarrhea. In other words, it is a “smart” drug aimed at HER2-driven cancer. When taken as a pill once daily, zongertinib has shown dramatic tumor-shrinking effects in clinical trials. For the patients who fit this profile (HER2-mutant, prior treatment), about 70–75% saw their tumors shrink significantly – meaning 7 or 8 out of 10 patients responded. Moreover, many of those responses lasted a long time (over half of responders were still responding at 6 months or more). Even patients who had already received another HER2-targeted therapy (such as trastuzumab deruxtecan) saw benefit: about 44% of them had tumor shrinkage. These numbers are very encouraging compared to older treatments.
Key Trial Results
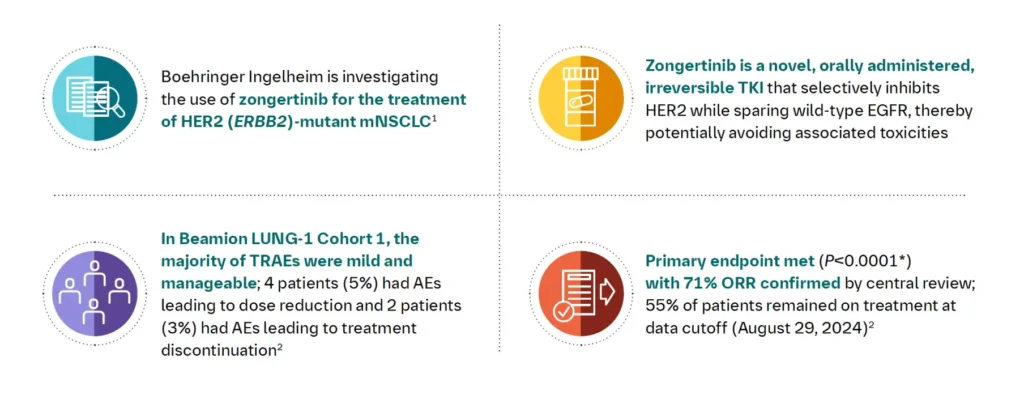
Clinical data behind the FDA’s decision came from the Beamion LUNG‑1 trial, a study of zongertinib in previously treated HER2-mutant NSCLC patients. In the main group of 71 patients (who had chemotherapy but no prior HER2 drugs), 75% responded and 58% had a response lasting ≥6 months. In the 34 patients who had also received a HER2-targeted antibody-drug (like deruxtecan), 44% responded and 27% had a response lasting ≥6 months. In plain language, most patients experienced tumor shrinkage, and for many this response lasted half a year or longer. Additional reports showed a median duration of response around 14 months and median progression-free survival over 12 months – meaning on average patients went about a year without disease worsening. For example, one analysis found the median time patients lived without cancer growth was about 17 months, which is very long for this setting.
These results are exciting because HER2-mutant lung cancers often behave aggressively and have few treatments. Zongertinib’s high response rate suggests it is a very active therapy for this small patient group. Importantly, brain metastases (cancer spread to the brain) also responded: roughly 40–60% of patients with brain lesions had shrinkage of their brain tumors, offering hope even for those with central nervous system involvement.
What This Means for Patients
This accelerated approval means that zongertinib can be prescribed to eligible patients immediately, even while confirmatory trials continue. In essence, it gives patients a new, approved option where none existed. Doctors now have a targeted therapy specifically for HER2-mutant lung cancer. Patients and families can feel hopeful that an innovative oral treatment is available. As one investigator noted, over 70% of patients “experienced a tumor response, which is highly meaningful”. Zongertinib could become the first oral HER2-directed therapy for lung cancer, transforming care for those who had exhausted other options.
Because it’s an “accelerated” approval, continued studies (a Phase III trial is ongoing) will confirm the long-term benefits. But for now, this is good news: it means the FDA believes the trial data show real benefit, and zongertinib will soon reach patients. The drug is taken once daily by mouth, with the dose based on body weight (patients under 90 kg take 120 mg; those 90 kg or above take 180 mg). It can be taken with or without food, and typically it’s continued until the cancer progresses or side effects become too much.
Possible Side Effects and Warnings
All cancer drugs have side effects, and zongertinib is no exception. In trials, most patients (about 80–95%) did have some side effects, but these were usually mild or moderate. The most common side effects were diarrhea (in roughly half of patients) and skin rash (about one-third to half). Other fairly common effects included nausea, fatigue, anemia (low red blood counts), decreased appetite, and occasional changes in liver tests. Most of these were low-grade. For example, in one analysis severe diarrhea occurred in only about 1% of patients and severe rash was essentially 0–2%.
Severe (Grade 3 or higher) side effects were seen in roughly 10–17% of patients. These were usually lab abnormalities, such as elevated liver enzymes. In the trial reports, only a very small number of patients had to stop treatment because of side effects (about 3% discontinued due to toxicity). Notably, no cases of treatment-related lung inflammation (pneumonitis) were seen in the zongertinib studies. This is reassuring, because another HER2 drug (trastuzumab deruxtecan) can cause this problem.
However, the official prescribing information includes important warnings. Patients will need regular monitoring of liver function, as zongertinib can cause liver damage (hepatotoxicity). Heart function should also be monitored because a small risk of heart muscle weakening (left ventricular dysfunction) exists. Lung health is watched for interstitial lung disease (pneumonitis) – though none was seen in trials, it can happen with many cancer drugs. And it’s critical to avoid pregnancy while taking zongertinib, as it can seriously harm a fetus. Doctors will discuss these risks and monitor closely.
Access and Next Steps
With FDA approval now granted, zongertinib will become available for patients in the coming months. Hospitals and cancer centers should be able to order the drug soon. One key factor is identifying the right patients: the FDA approved the Oncomine Dx Target Test as a companion diagnostic. This means doctors should test NSCLC tumors for the specific HER2 mutations (tyrosine kinase domain mutations) using this FDA-approved test. Any patient with advanced non-squamous NSCLC should discuss comprehensive genomic testing if not already done, because catching a HER2 mutation makes them eligible.
To get zongertinib, patients will work with their oncologist. If their test is positive for HER2 TKD mutation and they’ve tried at least one prior treatment, zongertinib could be prescribed. Insurance coverage will likely be available now that it is FDA-approved, but patients should talk with their healthcare team and insurance company. The manufacturer, Boehringer Ingelheim, may offer patient assistance programs (as they do with other medicines) to help with co-pays or for uninsured patients. Patients and caregivers should ask their doctors or nurses about financial support resources if cost is a concern.
In summary, zongertinib (Hernexeos) represents an exciting new option for patients with HER2-mutant NSCLC. It has shown high response rates and long-lasting benefit in clinical trials, and the FDA’s approval means doctors can now prescribe it. While side effects exist, they were generally manageable, and the most serious were infrequent. This oral therapy brings hope to a small group of lung cancer patients who until now had very limited choices. As one researcher put it, zongertinib “offers a new approach” and could be a “groundbreaking innovation for patients with limited treatment options”.
Key sources: FDA press release and news announcements (FDA.gov fda.gov), oncology news sites (OncNursingNews oncnursingnews.com, Cancer Network cancernetwork.com, OncoDaily oncodaily.com), and the published Beamion LUNG-1 trial data. These sources provided the approval details, trial results, and safety information summarized above.



Hello from Thailand
Can Her2 Exon20 patients residing in Thailand use it? What methods are available? Are there any research projects and pharmaceutical companies that we can contact?
Thank you for your question. Patients outside the US may be able to access Zongertinib through an early access program sponsored by the pharmaceutical company (Boehringer Ingelheim) or through a special prescription provided by an oncologist. Typically, the latter option is considered when no other treatment alternatives are available, and costs are likely to be paid out of pocket.
For the most accurate and up-to-date information, we recommend contacting Cancer Centers of Excellence or major hospitals in Thailand. These institutions should have detailed information regarding the availability of early access programs or alternative options for obtaining this medication.
You can read more information about early access program here: https://patientsavvy.org/early-access-program-for-non-approved-medicines-outside-the-us/