For patients battling metastatic colorectal cancer (mCRC), treatment often involves multiple strategies—chemotherapy, targeted therapies, immunotherapies, or local ablation methods like radiofrequency ablation (RFA) or microwave ablation. Yet in some cases, these options can fail, be ruled out due to tumor location, or cause unwelcome side effects.
Histotripsy is a non-invasive, non-thermal technology harnessing focused ultrasound to mechanically destroy tumor cells. Unlike heat-based approaches (like RFA), histotripsy spares healthy tissues and blood vessels, potentially improving safety and helping patients recover faster. Early studies and real-world experiences are showing that this approach can be a groundbreaking addition for patients with difficult-to-treat or otherwise inoperable liver lesions.
ULTRASOUND-MEDIATED TUMOR DISINTEGRATION
At its core, histotripsy directs high-energy ultrasound waves—typically in the range of 300 to 700 kPa—into a precisely targeted area of tumor tissue. When the waves converge at a focal point, they spark a phenomenon known as acoustic cavitation. During this process, the energy generates extremely small micro-bubbles that rapidly expand and implode with substantial force (often exceeding 10 MPa). The repeated implosion of these bubbles shatters tumor cells into subcellular fragments, most smaller than two micrometers.
Because this entire chain of events is purely mechanical, it avoids the thermal damage associated with procedures like radiofrequency ablation or microwave ablation. In organs such as the liver, where critical vessels and ducts are densely packed, the non-thermal nature of histotripsy helps protect these structures while precisely targeting tumor cells. Some patients are particularly good candidates if their lesions are close to large hepatic vessels or bile ducts—regions where heat-based techniques carry a higher risk of injury.
Immunomodulatory effects
One of the most intriguing aspects of histotripsy is its potential to trigger an immune response. Studies in preclinical models indicate that when tumor cells are broken apart by the cavitation process, they release damage-associated molecular patterns (DAMPs) such as HMGB1 and ATP. These molecules attract immune cells, including tumor-infiltrating lymphocytes (TILs) and natural killer (NK) cells, to the site of destruction. Some research even suggests that patients could experience an “abscopal effect,” where local treatment in one area leads to immune-mediated regression of distant, untreated tumors. Although these findings are still being validated in human trials, the possibility of mobilizing the body’s own defenses adds an appealing dimension to histotripsy’s value.
CLINICAL APPLICATION IN COLORECTAL LIVER METASTASES
Liver metastases are a major concern for patients with metastatic colorectal cancer (mCRC). More than half of individuals with advanced colon cancer will see tumors spread to the liver at some point. Histotripsy appears especially promising for small metastatic lesions—often up to about three or four centimeters in diameter—that are accessible to ultrasound. Because it spares healthy liver tissue and does not rely on heat, histotripsy can be an attractive choice for patients whose tumors are situated near vital blood vessels or ducts. Some clinical protocols also suggest that the procedure may shorten hospital stays, allowing patients to recover more quickly and potentially resume other treatments such as chemotherapy or immunotherapy.
Patient selection criteria
Before recommending histotripsy, physicians evaluate several factors to ensure safety and effectiveness. Clinical trials like #HOPE4LIVER typically enroll patients who have three or fewer liver metastases and individual tumor diameters of no more than three centimeters (some studies allow tumors slightly larger than four centimeters). Another key consideration is maintaining a safe margin from major bile ducts or large hepatic veins, generally at least one centimeter. Patients also need adequate blood clotting function, since any uncontrolled bleeding disorder poses a risk. Tumors located immediately next to gas-filled organs—such as the stomach or bowel—can be more challenging, because air disrupts the path of the ultrasound waves. Ultimately, a careful review of imaging scans (CT, MRI, or ultrasound) is critical for determining whether histotripsy is feasible.
Procedural workflow
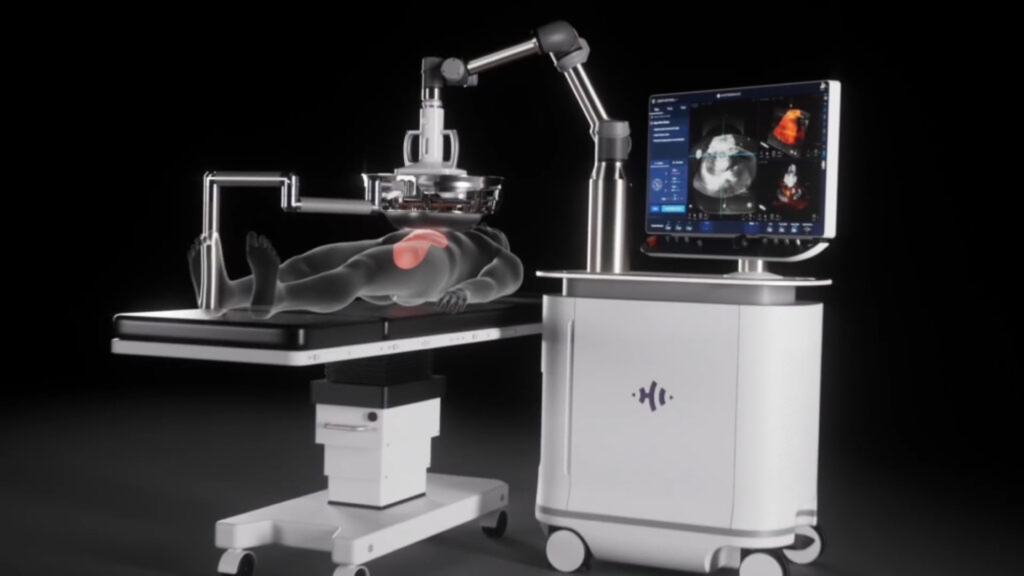
Planning usually starts with detailed imaging. Multiphase CT or MRI helps define tumor boundaries and account for movement related to breathing. During the actual procedure, the patient is placed under general anesthesia. Real-time ultrasound guidance allows the clinical team to direct the high-energy waves precisely onto the tumor. A single session often lasts between 45 and 90 minutes, depending on the number and size of lesions. Afterward, most patients are monitored for about 24 hours to ensure no immediate complications arise. A follow-up MRI or CT scan is generally performed within a day or two to verify that the tumor was adequately targeted and destroyed.
EFFECTIVENESS OF HISTOTRIPSY
Emerging data from clinical trials underscore the promise of histotripsy. In the multicenter #HOPE4LIVER trial, which enrolled 44 patients with various liver tumors (including metastases from colorectal cancer), investigators reported a technical success rate near 95 percent and a local tumor progression rate of only around 4.5 percent at six months. Major complications were relatively low, at about 6.8 percent, and the median hospital stay stood at just over one day. Notably, nearly 60 percent of the treated lesions in this study were metastases from colorectal primary tumors, and they showed outcomes comparable to primary liver cancers.
When compared to RFA, histotripsy does not rely on heat to kill cells. This distinction translates into several practical advantages, including a lower risk of damage to structures like major bile ducts that lie close to the targeted lesion. Although RFA remains a standard in many centers, complication rates in the published literature often range from 14 to 22 percent, which is notably higher than what histotripsy has shown in early studies. Some patients also experience mild post-procedural pain with histotripsy as opposed to moderate or severe discomfort commonly reported with RFA. Given these factors, many specialists see histotripsy as a potential “game-changer,” particularly for tumors adjacent to large vessels.
| Factor | Histotripsy | RFA |
|---|---|---|
| Mechanism | Non-thermal, mechanical | Thermal (heat) |
| Complication Rate | ~6.8% | 14–22% |
| Near-Vessel Safety | Safe <5 mm | Often discouraged |
| Immunologic Effect | Demonstrated | Minimal |
| Pain Profile | Mild | Moderate to severe |
REAL-LIFE PATIENT SUCCESS STORIES
Individual accounts from people who have undergone histotripsy for metastatic colorectal cancer highlight the real-world impact of this procedure. Mr. J, a 58-year-old patient with KRAS-mutant colon cancer, had two lesions in his liver that resisted shrinking on FOLFOXIRI chemotherapy. He underwent a single histotripsy session lasting just under 90 minutes. Over the next eight weeks, his tumor marker (CEA) plummeted from 784 ng/mL to 112 ng/mL. Subsequent PET/CT imaging showed no significant activity in those lesions. Nearly a year later, his liver remains free of recurrence, enabling him to continue systemic treatments for other disease sites as needed.
In another anecdotal example, a 62-year-old man named K. had two stubborn liver metastases that persisted through standard therapies. A one-time histotripsy procedure, performed on an outpatient basis, almost completely obliterated at least one tumor, leaving him with minimal pain and the ability to go home the very next day. Another patient, M., was initially concerned about thermal injury to a central bile duct. By participating in a trial that offered histotripsy, she avoided that risk, and follow-up imaging confirmed that around 95 percent of her target lesion had been destroyed—without harming the nearby duct.
LIMITATIONS AND RESEARCH FRONTIERS
While these early results are encouraging, it is important to consider several constraints. Histotripsy is most effective for tumors that measure three centimeters or less in diameter, although researchers are testing its feasibility in lesions up to about four centimeters. The presence of gas-filled structures, such as the stomach or portions of the bowel, can impede ultrasound transmission. Costs can also be higher compared to older methods like RFA, and insurance coverage varies depending on policy and location. The technique requires a specialized skill set, and centers that are just starting out with histotripsy may need more time per procedure while they build experience.
Ongoing trials are exploring whether histotripsy can be combined with systemic therapies, such as immunotherapy, to maximize any immune-mediated effects. The hope is that by creating robust tumor cell debris in the liver, the procedure might prime the body’s immune defenses to attack residual disease elsewhere. Although preliminary evidence suggests such an effect might exist, more data will be required to confirm and optimize this approach.
KEY QUESTIONS FOR YOUR HEALTHCARE TEAM
If you or a loved one is intrigued by histotripsy as a treatment option, it can help to ask your doctor whether the tumor’s size, location, and biology make you a suitable candidate. You might also want to discuss how this procedure fits into your broader treatment plan, particularly if you are undergoing chemotherapy or immunotherapy. It is also reasonable to inquire about short-term side effects and expected recovery time, as well as the possibility of enrolling in clinical trials that may offer additional benefits.
Where to find histotripsy in the United States
Because histotripsy is still in the early stages of adoption, it is generally available at select academic or comprehensive cancer centers, many of which are part of clinical research collaborations. Institutions like the University of Michigan, Henry Ford Health System, the Cleveland Clinic, the Mayo Clinic, and UVA Health System have led or participated in pivotal studies such as #HOPE4LIVER. Your oncologist may know of additional facilities that are either already performing histotripsy or are planning to start soon. Another option is to consult ClinicalTrials.gov by searching “histotripsy” or “#HOPE4LIVER” to find the latest information on open trials. Checking official provider websites, such as that of HistoSonics (one of the main developers of histotripsy equipment), may also reveal which hospitals have begun using the technology.
CONCLUSION
Histotripsy represents a truly innovative way to deal with metastatic colorectal cancer in the liver, offering a precise, non-thermal method of “mechanically shredding” malignant tissue while largely sparing the surrounding healthy structures. Early clinical outcomes have shown high technical success, low complication rates, and strong indications that histotripsy may foster an immune response against cancer. If you are facing metastatic colorectal cancer and have liver lesions in positions where other local therapies may carry unacceptable risks, histotripsy is worth discussing with your care team. Although the technique remains somewhat specialized and not yet widely available, its potential to transform local tumor ablation is generating a wave of optimism among both doctors and patients. As research continues and more centers gain expertise in delivering histotripsy, the hope is that this novel approach will become an integral part of the therapeutic arsenal for advanced colorectal cancer.
Sources
- Johns Hopkins Medicine (Histotripsy for Liver Tumors)
- Michigan Medicine (Histotripsy liver tumor trial successful, early clinical adoption recommended)
- The ASCO Post
Disclaimer: This content is for educational purposes only and should not be taken as medical advice. Always consult a qualified healthcare professional for personalized treatment decisions.