Introduction: From Gloomy Statistics to New Hope
Stage IV gastric cancer – meaning the cancer has spread to distant organs – was long associated with very bleak survival statistics. Traditionally, only around 4–10% of patients diagnosed at this advanced stage were still alive five years later. Such numbers, often cited from older data, painted a grim picture and understandably caused despair for patients and their loved ones. But the past decade has seen remarkable improvements. New treatments and smarter strategies are transforming what was once a nearly hopeless scenario into one with genuine hope and possibility. Today, leading oncologists urge patients not to be discouraged by outdated averages, because outcomes are improving every year and can vary widely depending on individual factors. In this article, we explore how 5-year survival rates in stage IV gastric cancer have significantly improved in recent years – and why patients have more reason to be optimistic than ever.

Dramatic Improvements in 5-Year Survival Rates
Recent data from research centers around the world show clear upward trends in survival for advanced gastric cancer:
- Higher Survival at Leading Centers: At one large medical center in China, the 5-year relative survival rate for stage IV gastric cancer reached 29% by 2018–2022. This is a huge improvement compared to prior years at the same center – in fact, it represents about a 15 percentage-point increase from the 2013–2017 period. Such gains reflect the impact of better treatments introduced in the last decade.
- Nationwide Trends: Broad population data also confirm progress. An analysis of U.S. cancer registry data noted that by the late 2010s, the predicted 5-year survival for distant-stage (metastatic) gastric cancer was around 10%. While still low, this is modestly higher than older statistics (often 5% or less) and is expected to continue rising. Similarly, a Japanese survey reported that with aggressive modern treatment, 5-year survival in stage IV can be boosted to about 16% – roughly four times higher than the historical average.
- Clinical Trial Outcomes: Perhaps most encouraging, clinical trials of new therapies show that some patients are doing far better than before. In a recent global trial of an immunotherapy drug (nivolumab) combined with chemotherapy as first-line treatment for advanced gastric/GE junction cancer, about 16% of patients were alive at 5 years, compared to only 6% of those who received older standard chemotherapy alone. Even across all patients in the trial (regardless of specific biomarkers), the addition of immunotherapy roughly doubled the 5-year survival rate (12% vs 6%). This kind of improvement was almost unheard of a decade ago in metastatic gastric cancer.
- Improvements Over Time: The pattern is clear – outcomes in stage IV gastric cancer have been improving year by year. Much of the commonly quoted survival data is based on patients diagnosed many years in the past, before the latest advances were available. As newer therapies (discussed below) take effect, the averages are steadily rising, and more patients are becoming long-term survivors. It’s important to realize that if you were diagnosed today, your prognosis may be far better than the “old” statistics suggest.
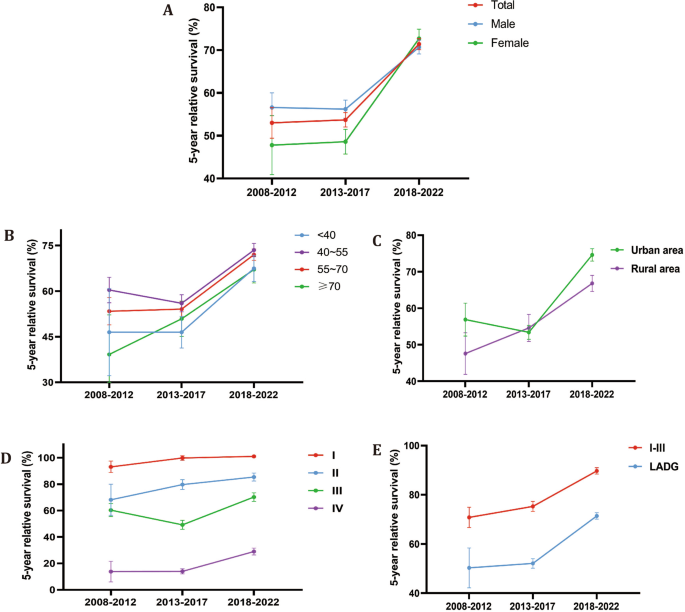
Figure: Long-term trends in 5-year survival for gastric cancer by stage (data from Nanfang Hospital, Guangzhou). Panel D shows the 5-year relative survival rates for stage I through IV. Notice the purple line for stage IV disease rising from the single digits in 2008–2012 to nearly 30% by 2018–2022. This dramatic jump reflects the impact of improved treatments and care over the past decade.
Not All Stage IV Cancers Are the Same
It’s also crucial to understand that stage IV gastric cancer encompasses a wide spectrum of situations, and survival can vary greatly depending on where the cancer has spread and other factors:
- Site of Metastasis: Gastric cancer can spread to different organs (liver, peritoneum (abdominal lining), lungs, distant lymph nodes, etc.). Some patterns carry a better outlook than others. For instance, if a patient has a single metastasis (say, only one spot in the liver or one ovary metastasis), they might do much better than someone with widespread disease. In certain cases, such patients can even be treated with curative intent (e.g., chemotherapy to shrink tumors followed by surgery to remove residual disease) – leading to long-term survival in a subset. On the other hand, diffuse spread, such as extensive peritoneal carcinomatosis (many tumor deposits throughout the abdominal lining), is more challenging, though even here new approaches like intraperitoneal chemotherapy (delivering chemo directly into the abdomen) and immunotherapy are being studied to improve outcomes.
- Tumor Biology & Histology: The biological subtype of the tumor also matters. Gastric cancers are often classified into intestinal-type or diffuse-type (including signet ring cell carcinoma). Diffuse-type cancers (especially linitis plastica) tend to be more aggressive and historically had lower survival. However, even among these, there are exceptions – for example, if a diffuse-type tumor is MSI-high or PD-L1 positive, it may respond well to immunotherapy. Intestinal-type cancers sometimes have more treatment options (like HER2-targeted therapy if HER2-positive). Researchers are also classifying gastric cancers by molecular profiles (the TCGA molecular subtypes) – such as EBV-positive, microsatellite unstable, genomically stable, etc. – which can inform therapy. The key point for patients is that knowing the subtype of your cancer can open doors to specific treatments that improve survival. Don’t hesitate to ask your doctor about molecular testing on your tumor – it could reveal a targetable alteration or a feature that makes immunotherapy more likely to work.
- Patient Health and Age: Patients who are younger or have fewer other health problems are often able to withstand more intensive treatments, which can translate into better outcomes. For example, a fit patient in their 50s might be a candidate for aggressive chemotherapy combinations, plus surgery and even radiation if needed, whereas a very frail patient might only manage gentler therapy. The survival statistics don’t always capture these distinctions. So, if you’re otherwise healthy and your cancer is biologically susceptible to modern treatments, your chances of long-term survival may be higher than the average.
- Time of Data Collection: As mentioned earlier, a lot of survival data is retrospective – meaning it looks at patients diagnosed in the past. With how fast treatment is evolving, even a 5-year-old statistic may no longer reflect current reality. For instance, the widespread use of immunotherapy in gastric cancer only started around 2017–2018 in many countries (after trials like ATTRACTION-2 and CheckMate-649 showed benefits). Patients treated since then have not all been followed for five years yet, so the official survival rates will likely climb as those patients are tracked. This lag in data is why many oncologists caution against taking older survival figures as destiny. We are charting new territory now – every year, more stage IV patients are beating the odds and living longer.
Bottom line: “Stage IV” is not a uniform verdict. There is a lot of variability and potential for positive outliers, especially given the new tools doctors have. It’s important for patients and caregivers to discuss the specifics of their case with the medical team – you may discover that your prognosis, with modern therapy tailored to your situation, is much better than you feared.
Innovations Fueling Better Outcomes
Let’s delve a bit more into the key innovations that are making these survival gains possible:
Immunotherapy – Unleashing the Immune System
The introduction of immunotherapy has arguably been the biggest game-changer for advanced gastric cancer in the last decade. Drugs known as immune checkpoint inhibitors (like nivolumab/Opdivo and pembrolizumab/Keytruda) help the body’s own immune system recognize and attack cancer cells. While not all patients benefit, a meaningful subset experiences major tumor shrinkage, prolonged disease control, and even complete remission in rare cases. For example, the nivolumab-plus-chemo trial mentioned earlier achieved a 60% response rate in patients with PD-L1 positive tumors, and some responses have proven to be very durable. Long-term follow-up confirms a tail of the survival curve – meaning a percentage of patients remain alive years out, effectively disease-free long after treatment, something virtually never seen with chemotherapy alone in the past.
Importantly, certain biomarkers predict higher success with immunotherapy. PD-L1 expression and MSI-high status are two such markers. Tumors that are MSI-high (caused by defects in DNA repair) tend to have lots of mutations and appear foreign to the immune system, so checkpoint inhibitors often work exceptionally well. In fact, one analysis found that adding immunotherapy in MSI-high metastatic gastric cancers reduced the risk of death by ~63% (HR ~0.37) compared to chemo. Even microsatellite-stable cancers can respond if they have high PD-L1 or other favorable immune features, as evidenced by case reports of complete remission in PD-L1 positive patients. Researchers are now testing immunotherapy combinations (like two checkpoint inhibitors together, or immunotherapy plus other agents) to further increase the fraction of patients who benefit.
For patients and caregivers, the takeaway is that immunotherapy offers real hope of extended survival for some stage IV gastric cancers. Doctors often check the tumor for PD-L1 and MSI (and sometimes tumor mutational burden or EBV) to gauge if immunotherapy is worth trying. Even if those markers are not present, immunotherapy might be used after other treatments, since some patients without obvious biomarkers still respond. The stories of patients who were gravely ill from metastatic gastric cancer and then bounced back after immunotherapy are inspiring – and increasingly common. (We’ll share an example in a later section.)
Targeted Therapies – Personalizing Treatment
Another leap forward has come from targeted therapies, which are drugs designed to attack specific molecular features of cancer cells:
- HER2-Targeted Therapy: About 1 in 5 advanced stomach cancers has too much of the HER2 protein on the cancer cell surface. For these, adding the monoclonal antibody trastuzumab (Herceptin) to chemo has been a standard since around 2010, after the ToGA trial showed it improves survival. This targeted drug “locks onto” HER2 receptors, blocking growth signals and marking the cell for immune attack. It improved median survival from roughly 11 months to 13–14 months in HER2-positive metastatic gastric cancer in trials, and some patients lived beyond 2 years – modest gains, but a start. Today, newer HER2-targeted drugs are pushing further: for example, trastuzumab deruxtecan (Enhertu) has shown potent activity in HER2-positive gastric cancer that has progressed after Herceptin, leading to deeper responses and longer control in those patients. HER2-positive status can thus change the treatment journey significantly, offering additional lines of therapy that can extend life.
- VEGF-Targeted Therapy: Another targeted drug used in gastric cancer is ramucirumab (Cyramza), which inhibits VEGFR-2 (a blood vessel growth factor receptor). It’s often used in the second-line setting (after initial chemo) to slow tumor blood vessel formation. While its impact on 5-year survival is less dramatic than immunotherapy, it has contributed to incremental improvements, helping some patients maintain disease control longer and bridging them to other treatments.
- Claudin 18.2 and Other Emerging Targets: Very recently, a new targeted therapy has made waves – an antibody called zolbetuximab targeting Claudin 18.2, a protein found in many gastric cancers. In a 2023 trial for advanced gastric/GEJ cancers that express Claudin18.2, adding zolbetuximab to chemo significantly improved survival (median OS ~18 months vs 15 months with chemo alone) – a notable advance. While we don’t have 5-year data yet, this could further raise long-term survival as this drug becomes available. Researchers are also exploring targets like FGFR2b (with bemarituzumab) and others for subgroups of gastric cancer. Each new targeted agent might benefit a slice of patients, but collectively they ensure more patients have an effective option beyond standard chemo.
- Precision Medicine and Genomics: Beyond approved targets, some patients pursue comprehensive genomic profiling of their tumor to find any “druggable” mutations (e.g., in genes like MET, NTRK, etc.). While such mutations are relatively rare in gastric cancer, if found, they can sometimes be treated with specialized drugs (often via clinical trials). This is another reason not to rely solely on broad statistics – an uncommon genetic quirk in your cancer could make a big difference in your personal outcome if there’s a therapy for it.
Optimized Chemotherapy & Multimodal Care
Chemotherapy itself has improved over the decades. Newer chemo combinations (like FOLFOX, CapeOx, FLOT, etc.) are more effective and sometimes better tolerated. For example, the regimen known as FLOT has become a preferred option in some regions for advanced gastric cancer because it showed better efficacy. These refinements contribute to more patients responding and potentially living longer. In addition, doctors have learned to use maintenance therapies or sequential treatments to keep the cancer in check for as long as possible, turning it into more of a chronic illness in some cases.
The concept of multimodal therapy – using a combination of treatments like chemo, radiation, surgery, targeted drugs, and immunotherapy – has taken hold in advanced gastric cancer management. By attacking the disease from multiple angles, the chances of significantly shrinking it and controlling it improve. For instance, some patients with limited metastases might receive chemotherapy plus HER2-targeted therapy to shrink tumors, then undergo surgery to remove remaining disease, and later get immunotherapy as an adjuvant (additional) treatment. This kind of comprehensive plan can be complex, but when successful, it may yield outcomes once deemed impossible for stage IV disease (including the holy grail: No Evidence of Disease (NED) status – essentially remission).
Crucially, these aggressive approaches are only undertaken when there’s a reasonable likelihood of benefit. Oncologists use the initial response to treatment as a guide: if a metastatic tumor shrinks dramatically on chemotherapy or immunotherapy, it signals a biology that might allow for a surgical strike. Good responders are the ones who may become long-term survivors, and now we have more tools to induce responses than before. As one study noted, “patients with stage IV gastric cancer benefit most when they receive chemotherapy and have well-differentiated tumors” in the context of surgery – basically, when the cancer is biologically less aggressive and chemo-sensitive, extraordinary outcomes can follow. Identifying those cases and treating them optimally is a major focus of current research.
Real-Life Survivors: Stories of Complete Responses
Perhaps the most uplifting evidence of progress comes from individual patient stories – those rare but increasing cases where stage IV gastric cancer has been beaten into remission. These stories show what is possible and keep hope alive that “complete response” (disappearance of all cancer signs) does not have to be a myth. Here are a couple of examples from recent medical reports:
- Multiple Liver Metastases – Now Cancer-Free: Doctors in Guangzhou, China, reported the case of a 66-year-old man with stage IV gastric cancer that had multiple metastases to the liver. His situation was dire; normally, such patients have a very short life expectancy. He underwent an emergency surgery to remove his stomach (a palliative gastrectomy to control bleeding), and afterwards received several cycles of chemotherapy (SOX regimen: S-1 oral chemo plus oxaliplatin) followed by combined immunotherapy (nivolumab) with chemo. The results were astonishing: the patient’s tumors progressively shrank and eventually no evidence of cancer remained on scans. He achieved a complete remission. According to the report, he had no recurrence for 32 months after the treatment and surgery – that’s nearing three years of being cancer-free, a result practically unheard of for metastatic gastric cancer in the past. The doctors noted this was the first ever reported case of its kind (multiple liver mets cleared by immunotherapy after surgery) and discussed how the patient’s tumor characteristics (he was PD-L1 positive and had certain genetic mutations) might have contributed to the exceptional response. This case shines a light on the fact that immunotherapy, even though it helps only a subset, can sometimes completely turn things around.
- Peritoneal Spread – Long-Term Survival with Surgery and Immunotherapy: In Japan, a 73-year-old woman was diagnosed with stage IV gastric cancer with extensive peritoneal metastasis (tumor spread throughout the abdominal lining). She went through multiple lines of chemotherapy which initially kept the disease at bay, but then the cancer started worsening again. As a third-line treatment, she received nivolumab (immunotherapy), and notably, her tumor was MSI-high, a biomarker predicting good response to immunotherapy. The cancer responded dramatically – scans showed a complete clinical response after 24 cycles of nivolumab. However, after extended treatment, there were signs the primary tumor might be regrowing, so the medical team decided to operate. They performed a gastrectomy (removal of part of her stomach) and confirmed that no viable cancer was left in most areas (indicating a pathological near-complete response). After surgery, she continued on a year of post-op chemo as a precaution. According to the report, she had no recurrence at 39 months (over 3 years) after the surgery. This case exemplifies “conversion therapy” – using drug treatment to convert an initially inoperable cancer into a operable one – and it underscores how an MSI-high tumor can be rendered almost completely harmless by immunotherapy. The patient went from having a belly full of cancer implants (normally a fatal condition) to living years without any sign of disease.
These are just two cases, and each patient’s story is unique. Not every individual will have such a remarkable outcome. However, the fact that complete and lasting responses are being documented at all in stage IV gastric cancer is cause for optimism. Ten years ago, such reports were virtually nonexistent; now, with new therapies, they are beginning to surface in medical literature. They remind us that statistics are not destiny – some patients do far better than expected, and the ranks of these long-term survivors are growing as treatments improve.
For patients and families, reading about these successes can provide hope and questions to discuss with your oncologist. Could immunotherapy work for me? Am I a candidate for an aggressive approach? Even if the answer isn’t clear-cut, being aware of these possibilities can empower patients to seek second opinions or clinical trials, and to fight on knowing that extraordinary outcomes, while not guaranteed, are achievable.
Conclusion: Turning the Tide in Advanced Gastric Cancer
The journey through stage IV gastric cancer is undoubtedly challenging, and the road can be long and hard. Yet, unlike in the past, today there is a genuine reason to hold onto hope. The five-year survival rate for metastatic gastric cancer, once stuck in the single digits, has been steadily climbing thanks to relentless progress in oncology. We now have patients reaching that 5-year mark and beyond, cancer under control – something that used to be exceedingly rare. As data from recent years shows, many factors drive these survival improvements: smarter use of surgery, more effective drug combinations, targeted and immune therapies that didn’t exist a decade ago, and a more personalized approach to each patient’s tumor.
If you or your loved one is facing stage IV gastric cancer, remember that statistics are averages, and often outdated ones at that. Medicine is advancing fast. The “average” outcome from five or ten years ago may not apply in the era of immunotherapy and targeted treatment. Doctors now speak of potentially curative outcomes in selected metastatic cases – words that would have sounded like science fiction not long ago. While we must be realistic (most patients still do not achieve a cure, and much work remains to be done), we should also be encouraged that the needle is moving in the right direction.
Hope is very real, and it’s backed by data: survival curves inching upward, new therapies coming to clinics, and lives being extended. Every extra month and year gained represents precious time for patients to spend with their families, to pursue meaningful activities, and to possibly reach the next breakthrough. Researchers worldwide are continuing to refine treatments and are even exploring cutting-edge ideas like personalized cancer vaccines and cell therapies for gastric cancer. The momentum gives us confidence that the next decade will bring even better news.
Above all, remember that you are not a statistic. Every person’s cancer is different, and with the rapid improvements in care, there are valid reasons to remain optimistic. The significant rise in 5-year survival rates in stage IV gastric cancer over the last decade shows what can be achieved. It’s a testament to human resilience and scientific progress. By staying positive and proactive, patients and caregivers can ride this wave of progress, confident that each year brings new opportunities and that hope truly is on the horizon.
Sources: Recent clinical studies and reviews have been referenced to support the data and examples provided, including survival analyses frontiersin.org bmccancer.biomedcentral.com, clinical trial results, and published case reports of remarkable outcomes surgicalcasereports.springeropen.com. These peer-reviewed sources underline the factual improvements and hope-inspiring cases discussed in the article.


