Colorectal cancer (CRC) is one of the most preventable cancers, especially when detected early. Screening is a powerful tool that helps identify cancer or precancerous polyps before symptoms appear, giving patients and caregivers a fighting chance to stop the disease in its tracks. While colonoscopy has long been considered the gold standard for CRC screening, non-invasive options like Cologuard and its enhanced version, Cologuard Plus, are gaining attention for their convenience and effectiveness. In this blog post, we’ll explore how these tests work, their accuracy, limitations, and whether they can replace traditional screening methods like colonoscopy or older stool tests such as FOBT.
What Are Cologuard and Cologuard Plus?
Cologuard and Cologuard Plus are FDA-approved at-home stool tests designed to screen adults aged 45 and older who are at average risk for colorectal cancer. These tests analyze stool samples to detect DNA markers and traces of blood that may indicate the presence of colorectal cancer or advanced precancerous polyps. With no need for invasive procedures or sedation, these tests offer a convenient alternative for individuals who may feel hesitant about undergoing a colonoscopy.
Cologuard Plus builds upon the original test by improving sensitivity for detecting colorectal cancer and precancerous lesions while reducing false positives. This means fewer unnecessary follow-up procedures while maintaining high accuracy.
How Do These Tests Work?
Using Cologuard or Cologuard Plus is simple. Patients receive a kit to collect a stool sample at home, which is then mailed to a laboratory for analysis. The tests use advanced technology to search for hidden blood in the stool as well as specific DNA changes associated with colorectal cancer or precancerous polyps. These genetic mutations and epigenetic markers are often present in cells shed by tumors or abnormal growths into the digestive tract.
Compared to older stool-based tests like fecal occult blood testing (FOBT) or fecal immunochemical testing (FIT), Cologuard stands out because it doesn’t rely solely on detecting blood. By incorporating DNA analysis, it offers greater sensitivity, meaning it’s better at identifying cancers and precancerous conditions—even when lesions aren’t actively bleeding.
Why Are These Tests Not Suitable for High-Risk Individuals?
While Cologuard and Cologuard Plus are excellent options for average-risk individuals, they are not recommended for people at high risk of colorectal cancer. High-risk patients include those with a personal history of CRC or adenomas, inflammatory bowel disease (IBD), hereditary syndromes like Lynch syndrome, or a family history of colorectal cancer. These individuals require more precise monitoring tools like colonoscopy.
The reason lies in the limitations of stool-based tests. While they are effective at detecting cancer and advanced adenomas in average-risk patients, they may miss smaller or less developed lesions that could be significant in high-risk individuals. Colonoscopy allows direct visualization of the colon lining and enables doctors to remove polyps during the procedure, providing both diagnosis and prevention in one step—something stool-based tests cannot do.
Understanding False Positives and False Negatives
No screening test is perfect, including Cologuard. While it offers high accuracy, it can occasionally produce false positives or false negatives. A false positive occurs when the test suggests cancer or precancerous growths that aren’t actually present. This can lead to unnecessary anxiety and follow-up procedures like colonoscopy. For example, the original Cologuard has a false positive rate of about 13%, though this rate is significantly reduced with Cologuard Plus.
False negatives happen when the test fails to detect cancer or precancerous lesions that are truly present. Although rare with these tests—Cologuard Plus misses only about five out of every 100 cases of colorectal cancer—it’s important to remember that no screening method can guarantee 100% accuracy.
Patients who receive a positive result from either test must undergo a colonoscopy for confirmation. Similarly, those with negative results should continue regular screenings as recommended by their healthcare provider.
What Is the Role of Cologuard and Cologuard Plus in CRC Screening?
Can These Tests Replace Colonoscopy?
While Cologuard and Cologuard Plus provide valuable screening options, they cannot fully replace colonoscopy. Colonoscopy remains the gold standard because it not only detects abnormalities but also removes polyps during the procedure, preventing them from developing into cancer. Stool-based tests like Cologuard can identify potential issues but require follow-up colonoscopy for diagnosis and treatment.
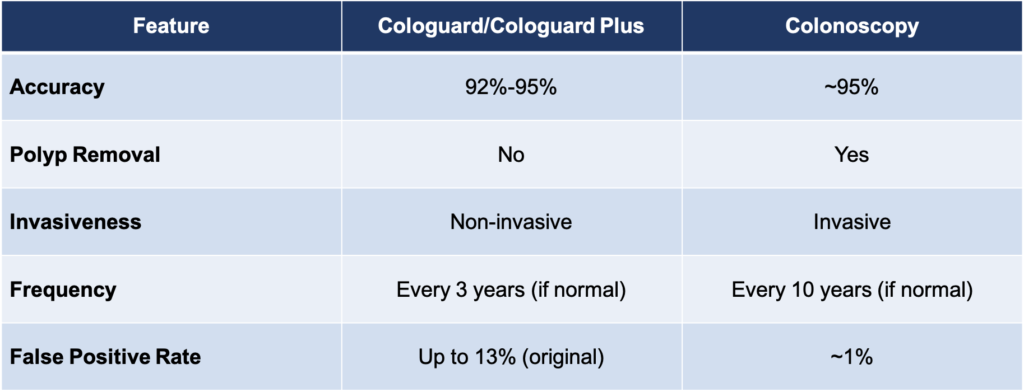
For average-risk patients who may be reluctant to undergo an invasive procedure or have medical conditions that make colonoscopy difficult, Cologuard offers an effective alternative that promotes adherence to screening guidelines. However, high-risk patients should always opt for colonoscopy due to its precision and preventive capabilities.
How Does Cologuard Compare to FOBT/FIT?
Compared to older stool tests like FOBT or FIT, which detect hidden blood in the stool as their sole marker, Cologuard offers significant advantages. By analyzing DNA mutations alongside blood markers, it provides higher sensitivity and fewer false negatives. For example, while FOBT detects about 67%–74% of colorectal cancers, Cologuard identifies 92%–95%. Similarly, advanced adenomas—precancerous growths—are detected in only 23%–24% of cases with FOBT but up to 49% with Cologuard Plus.
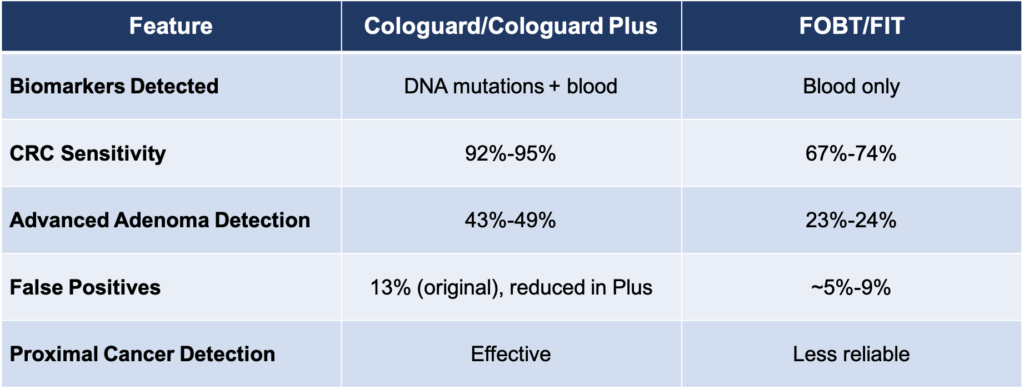
Older tests also struggle with detecting cancers located in the proximal (right-sided) colon because these lesions often don’t bleed consistently. In contrast, Cologuard’s DNA analysis improves detection rates across all parts of the colon.
Although FOBT/FIT remain lower-cost options in some healthcare systems or for patients prioritizing affordability over sensitivity, Cologuard’s advanced technology makes it a preferred choice for non-invasive screening among average-risk individuals.
Conclusion: Choosing the Right Screening Option
Cologuard and Cologuard Plus represent significant advancements in non-invasive colorectal cancer screening. They offer convenience without compromising accuracy and are ideal for average-risk individuals seeking an alternative to traditional methods like colonoscopy or older stool-based tests such as FOBT. However, they are not suitable for high-risk patients who require more precise monitoring tools like colonoscopy.
For patients and caregivers navigating screening options, understanding these differences empowers informed decision-making about colorectal cancer prevention. Early detection saves lives—whether through stool-based tests or colonoscopy—so choose the method that works best for your circumstances while following your healthcare provider’s recommendations.
Source: Exact Sciences; Cologuard HCP; Targeted Oncology.