Bevacizumab (Avastin) has transformed cancer treatment since its approval in 2004, offering new hope to patients with advanced cancers. This targeted therapy works by inhibiting vascular endothelial growth factor-A (VEGF-A), a key protein that fuels tumor growth by promoting new blood vessel formation. By blocking VEGF-A, bevacizumab cuts off a tumor’s blood supply, slowing its progression and, in some cases, enhancing the effectiveness of chemotherapy. While its clinical benefits are well-documented, bevacizumab’s journey highlights both the promise of innovation and the challenges of managing treatment-related risks.
How Bevacizumab Works: Starving Tumors of Their Lifeline
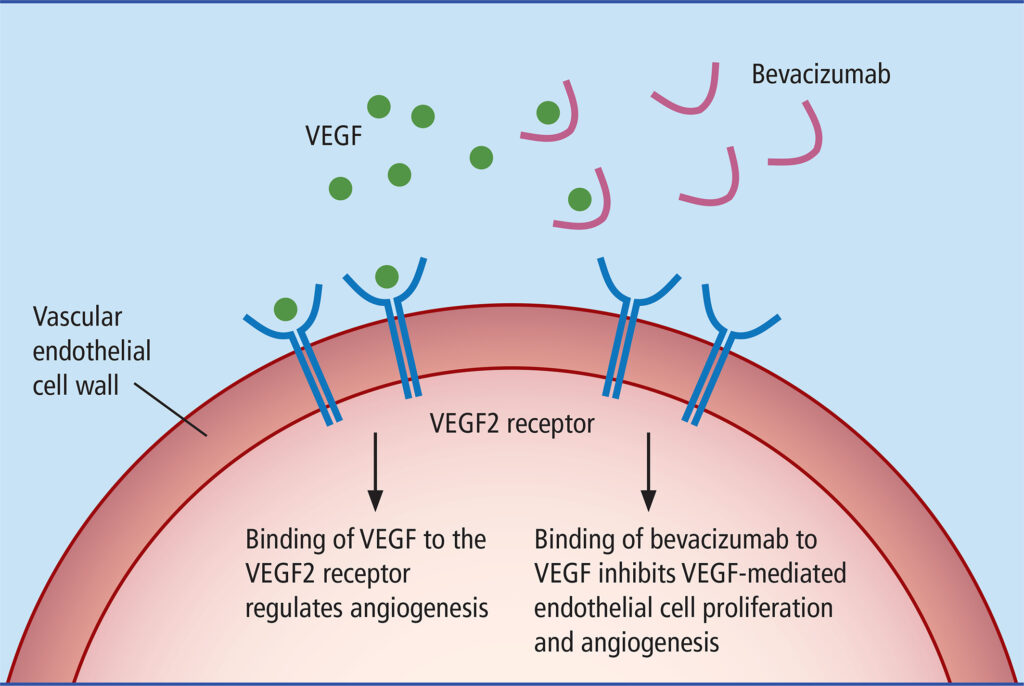
Tumors need a steady blood supply to grow and spread. Under low-oxygen conditions, they release VEGF to stimulate the development of fragile, leaky blood vessels. Bevacizumab works by neutralizing VEGF-A before it can attach to its receptors on blood vessel cells. This not only restricts the tumor’s access to nutrients but also stabilizes the remaining blood vessels, allowing chemotherapy drugs to penetrate the tumor more effectively.
Because of its large molecular size (>149 kDa), bevacizumab is administered intravenously. Once in the bloodstream, it binds to more than 97% of circulating VEGF, preventing it from stimulating new blood vessel growth. Pharmacokinetic studies have shown that bevacizumab reaches peak concentrations within 2.5 hours of infusion, with a sustained effect that necessitates intermittent high-dose regimens to maintain VEGF suppression. These properties make it an effective but complex treatment that requires careful dosing schedules.
A Breakthrough in Oncology: Bevacizumab’s Expanding Role
Initially approved for metastatic colorectal cancer (mCRC), bevacizumab quickly gained recognition across various cancer types. Over the years, regulatory approvals expanded to include non-small cell lung cancer (2006), renal cell carcinoma (2009), glioblastoma (2009), and ovarian cancer (2014). Clinical trials played a key role in this expansion.
For example, the RIBBON-1 trial demonstrated progression-free survival (PFS) improvements in breast cancer patients treated with bevacizumab. However, overall survival (OS) benefits remained inconclusive, leading to its withdrawal for metastatic breast cancer in the United States. Despite this, trials such as the SUNLIGHT study in mCRC continued to validate bevacizumab’s role. This 2023 study showed that combining bevacizumab with trifluridine/tipiracil (Lonsurf) extended median OS from 7.5 to 10.8 months in heavily pretreated patients, marking a milestone in late-stage mCRC management.
Clinical Applications: Tumor-Specific Outcomes
Colorectal Cancer: From First-Line to Salvage Therapy
In mCRC, bevacizumab has been incorporated into multiple lines of therapy:
- First-line treatment: When added to chemotherapy regimens like FOLFOX or FOLFIRI, bevacizumab improves PFS by approximately 4.7 months.
- Second-line treatment: Bevacizumab continues to provide benefits even when switching chemotherapy backbones.
- Third-line and beyond: The SUNLIGHT trial confirmed that bevacizumab in combination with Lonsurf reduces the risk of death by 44% compared to Lonsurf alone.
Glioblastoma: Addressing an Unmet Need
For recurrent glioblastoma, bevacizumab is often used to improve symptom management rather than as a curative therapy. Patients receiving bevacizumab monotherapy achieved six-month PFS rates of 42.6%, with notable reductions in cerebral edema and steroid dependence. While survival benefits remain modest, many patients experience improved neurological function and quality of life.
Ovarian Cancer: Delaying Progression
Patient testimonials highlight the role of bevacizumab in stabilizing ovarian cancer. One survivor shared, “After 12 rounds of Avastin, my CA-125 dropped to 10–14. Though I developed proteinuria requiring discontinuation, it bought me precious time.” Clinical trials like GOG-0218 demonstrated that adding bevacizumab to carboplatin and paclitaxel improved PFS by 3.4 months, reinforcing its role in maintenance therapy.
Patient Experiences: The Trade-Off Between Benefit and Side Effects
For many patients, bevacizumab offers a lifeline. A 65-year-old woman with ovarian cancer saw her tumor shrink by 65% after four treatment cycles, allowing her to maintain her daily activities despite the fatigue. Others, like glioblastoma survivors, report improved neurological function and better symptom control.
However, like any treatment, bevacizumab comes with challenges. A meta-analysis of 16 trials revealed a 50% increased risk of fatal events with bevacizumab (2.5% vs. 1.7%), particularly in combination with platinum- and taxane-based chemotherapies. Common severe side effects include:
- Hemorrhage: Responsible for 23.5% of treatment-related fatalities.
- Gastrointestinal perforation: Reported in 8.9% of fatal cases.
- Hypertension: Occurs in 8–18% of patients at grade 3/4 severity.
Despite these risks, patient-reported outcomes suggest a complex trade-off. In a study of 59 mCRC patients, pain, nausea, and diarrhea worsened during treatment, but emotional and cognitive function improved—likely due to disease control.
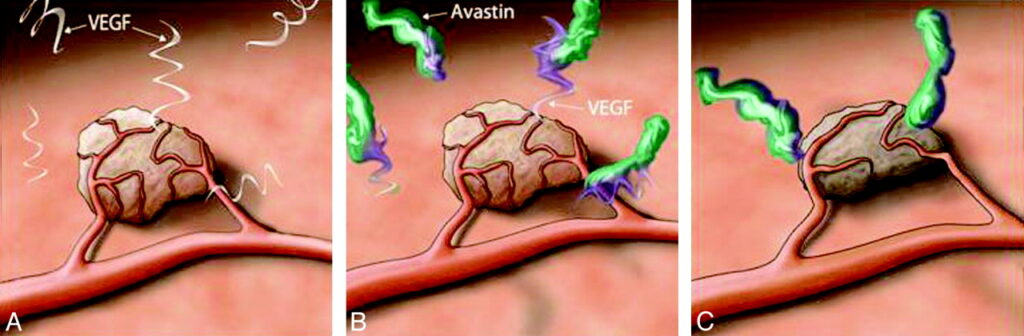
Optimizing Treatment: Strategies for Maximizing Benefit and Minimizing Risk
To improve outcomes, researchers are identifying biomarkers that predict which patients will benefit most from bevacizumab. Promising approaches include:
- Circulating VEGF-A levels: Potentially indicating treatment response.
- VEGF receptor polymorphisms: Genetic variations that may predict efficacy.
- Dynamic contrast-enhanced MRI: Used to assess blood vessel normalization in real time.
Managing side effects is also crucial. Common strategies include:
- Hypertension management: Early initiation of ACE inhibitors or calcium channel blockers.
- Proteinuria monitoring: Monthly urinalysis with treatment adjustments as needed.
- Bleeding risk mitigation: Avoiding use in tumors near major blood vessels or in patients with a history of severe hemorrhage.
The Future of Bevacizumab: Next-Generation Approaches
Combination Immunotherapy
Emerging research suggests that VEGF inhibition can enhance immune responses against tumors. The phase II FIGHT trial demonstrated promising results by combining bevacizumab with PD-1 inhibitors, showing a 65% response rate in hepatocellular carcinoma. Ongoing studies aim to refine these combination strategies.
Biosimilars and Cost Accessibility
The introduction of bevacizumab biosimilars, such as TAB008, has the potential to improve global access to this life-extending treatment. With over 10 biosimilars in development, cost reductions could make bevacizumab more widely available to patients worldwide.
Conclusion: A Powerful Tool in Cancer Care
Bevacizumab has reshaped cancer treatment, offering many patients longer survival and better disease control. However, it also underscores the need for careful treatment planning, balancing benefits with potential risks. As research progresses, the goal remains clear: refining treatment strategies, improving patient selection, and ensuring that each person receives the most effective care possible.
For patients and caregivers, staying informed and engaged in treatment decisions is essential. While bevacizumab is not a cure, it continues to provide valuable time—time that many patients cherish as they fight their disease and spend moments with loved ones. The future holds promise, and with ongoing advances, the role of angiogenesis inhibitors like bevacizumab will only continue to evolve.
Source: DrugBank, National Cancer Institute, PubMed Central

Pingback: Stage IV Colon Cancer Survival: A Decade of Dramatic Improvement and Hope